Cell Chemical Biology: CDK2 heterobifunctional degraders co-degrade CDK2 and cyclin E resulting in efficacy in CCNE1-amplified and overexpressed cancers
Cell Chemical Biology: CDK2 heterobifunctional degraders co-degrade CDK2 and cyclin E resulting in efficacy in CCNE1-amplified and overexpressed cancers
Nicholas Kwiatkowski, Tong Liang, Zhe Sha, Philip N. Collier, Annan Yang, Murugappan Sathappa, Atanu Paul, Lijing Su, Xiaozhang Zheng, Robert Aversa, Kunhua Li, Revonda Mehovic, Christina Kolodzy, Susanne B. Breitkopf, Dapeng Chen, Charles L. Howarth, Karen Yuan, Hakryul Jo, Joseph D. Growney, Matthew Weiss, Juliet Williams
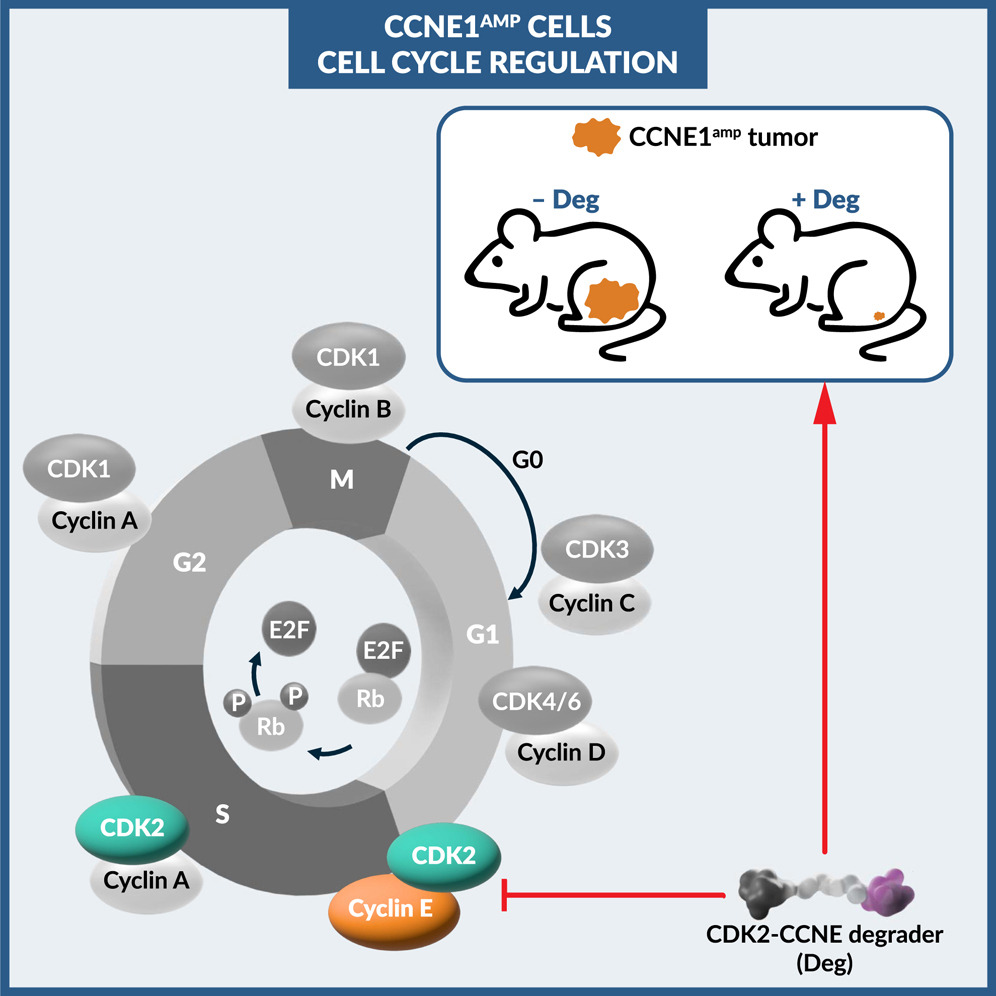
Amplification and overexpression of CCNE1, which encodes cyclin E1, is a common event in tumor cells and occurs with high frequency in ovarian and endometrial cancers. Tumors with CCNE1 amplification show resistance to first-line chemotherapies and investigational poly (ADP-ribose) polymerase (PARP) inhibitors, and cyclin E1 (and E2) overexpression is associated with resistance to CDK4/6-inhibitor–endocrine combination therapy in HR+/HER2-metastatic breast cancer. These examples highlight a significant unmet medical need in patients with cyclin E-driven tumors. In tumor cells with CCNE1 amplification, cyclin E overexpression leads to aberrant activation of its catalytic partner CDK2, resulting in dysregulated cell cycle control and a growth advantage for tumor cells. While research has identified CDK2 as a rational therapeutic target for eliminating these tumors, clinical translation has been challenging as small-molecule inhibitors targeting CDK2 frequently inhibit other CDK family members including CDK1, which is essential for normal cell division, resulting in a narrow therapeutic window with these agents. We developed a compound that employs targeted protein degradation (TPD) technology to achieve selective removal of CDK2 while sparing CDK1. In the course of our work we discovered that this approach also results in the degradation of cyclin E1, a protein previously considered “undruggable”. We demonstrate that the combined degradation of CDK2 and cyclin E1 effectively suppresses the oncogenic pathway in both CCNE1-amplified tumors and in cancer cells resistant to CDK4/6 inhibitors. Simultaneously affecting both the kinase and its regulatory partner may provide robust pathway suppression and help address challenges associated with targeting CDK2 with traditional inhibitors. These findings suggest TPD could be an important advancement in the field of biomarker-guided cancer treatments; however, further development is required to determine potential therapeutic applications.