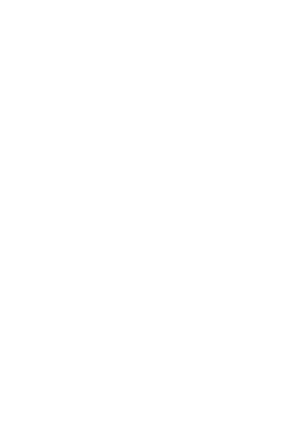Kymera is at the forefront of a new technology called targeted protein degradation that offers us a new way to treat disease.

Targeted Protein Degrader Medicines Work with Your Cells to Fight Disease
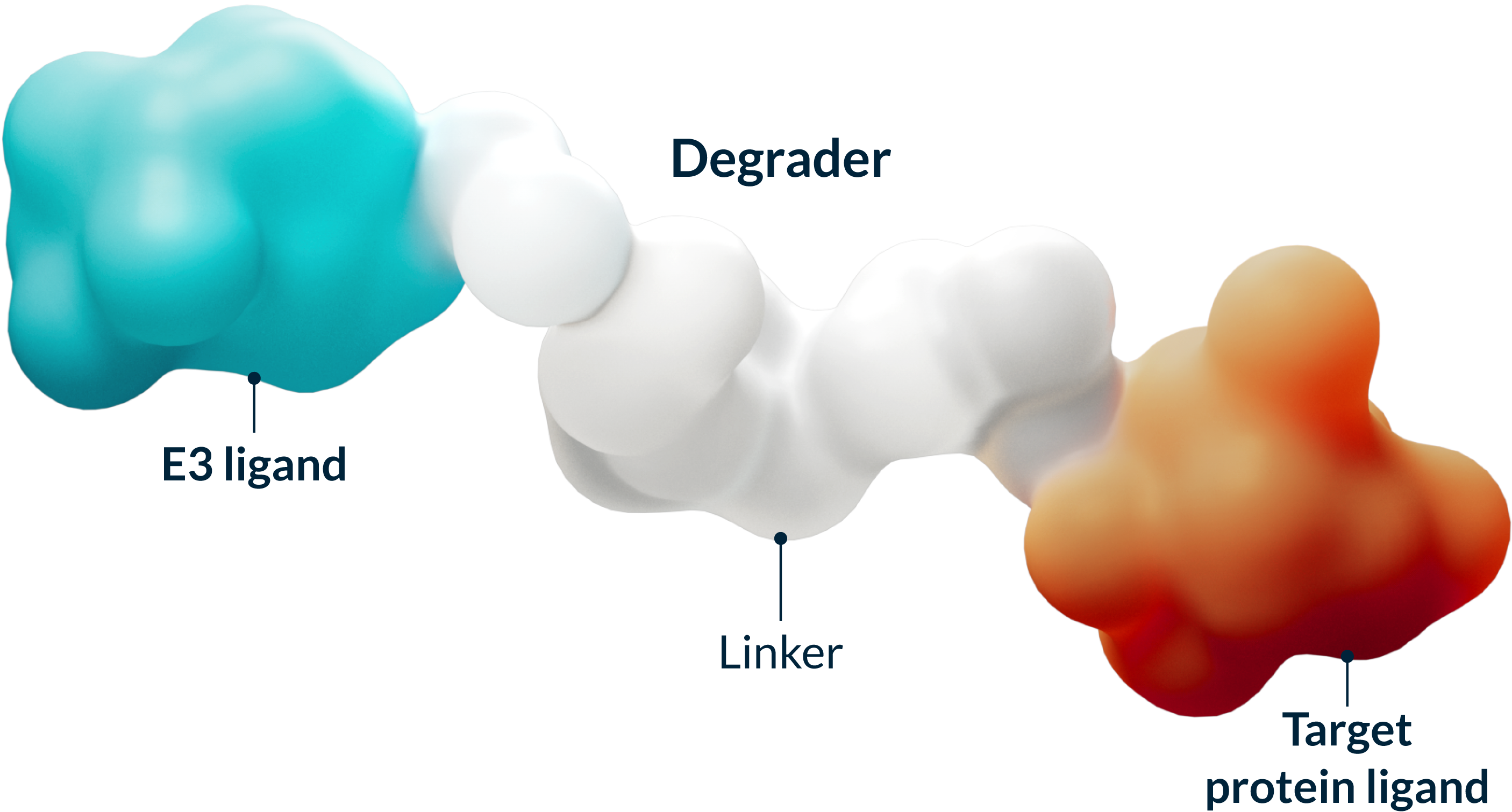
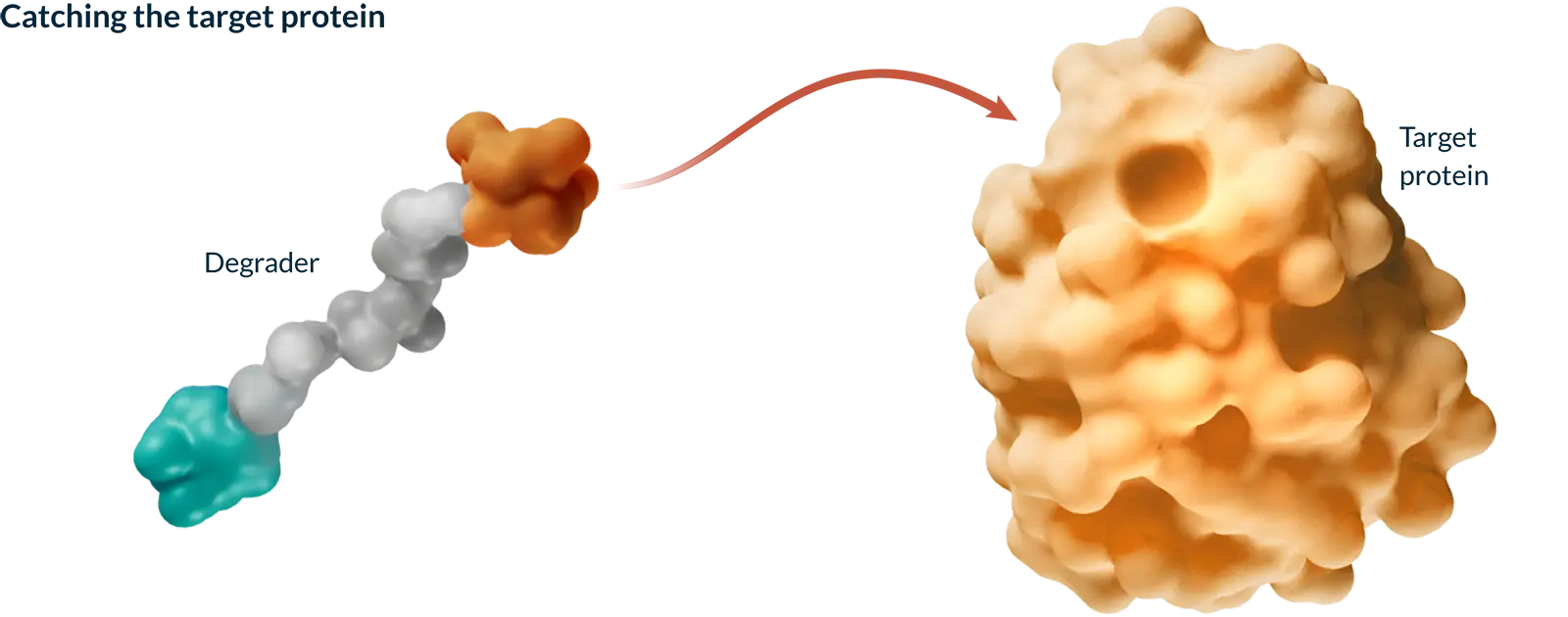
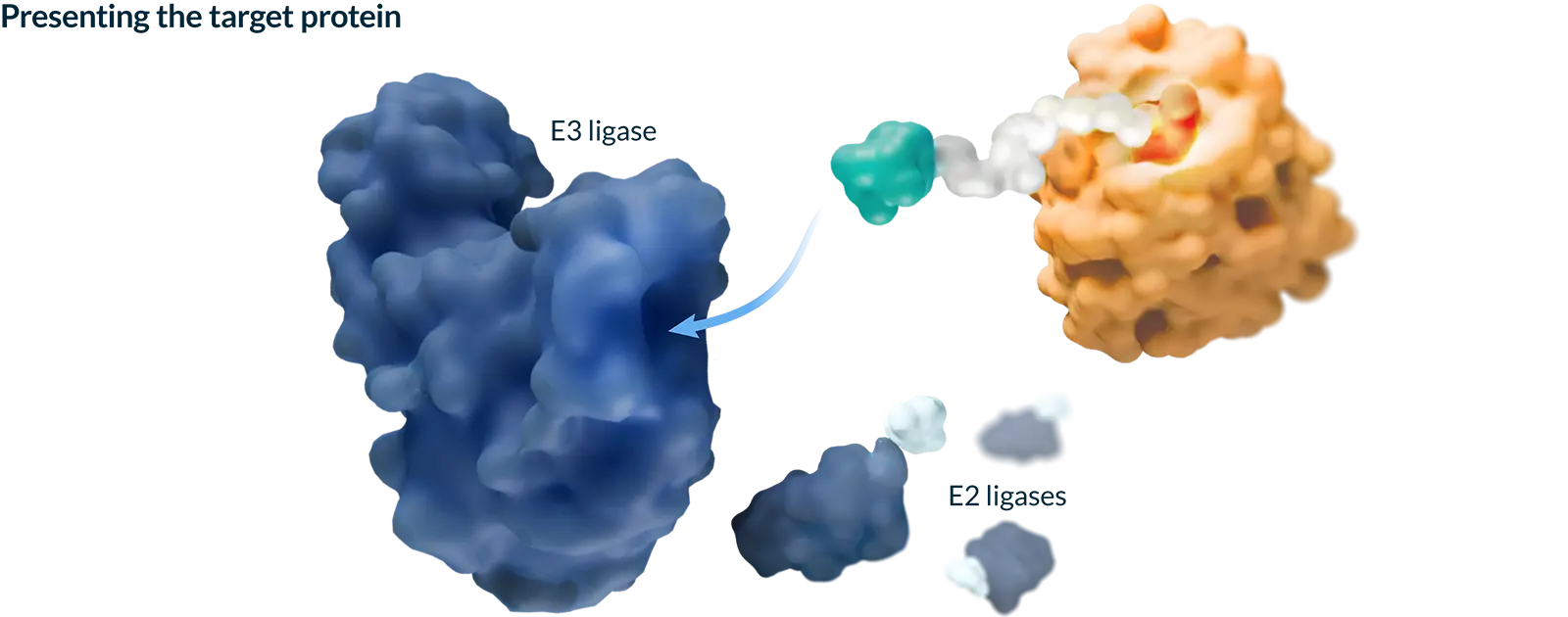
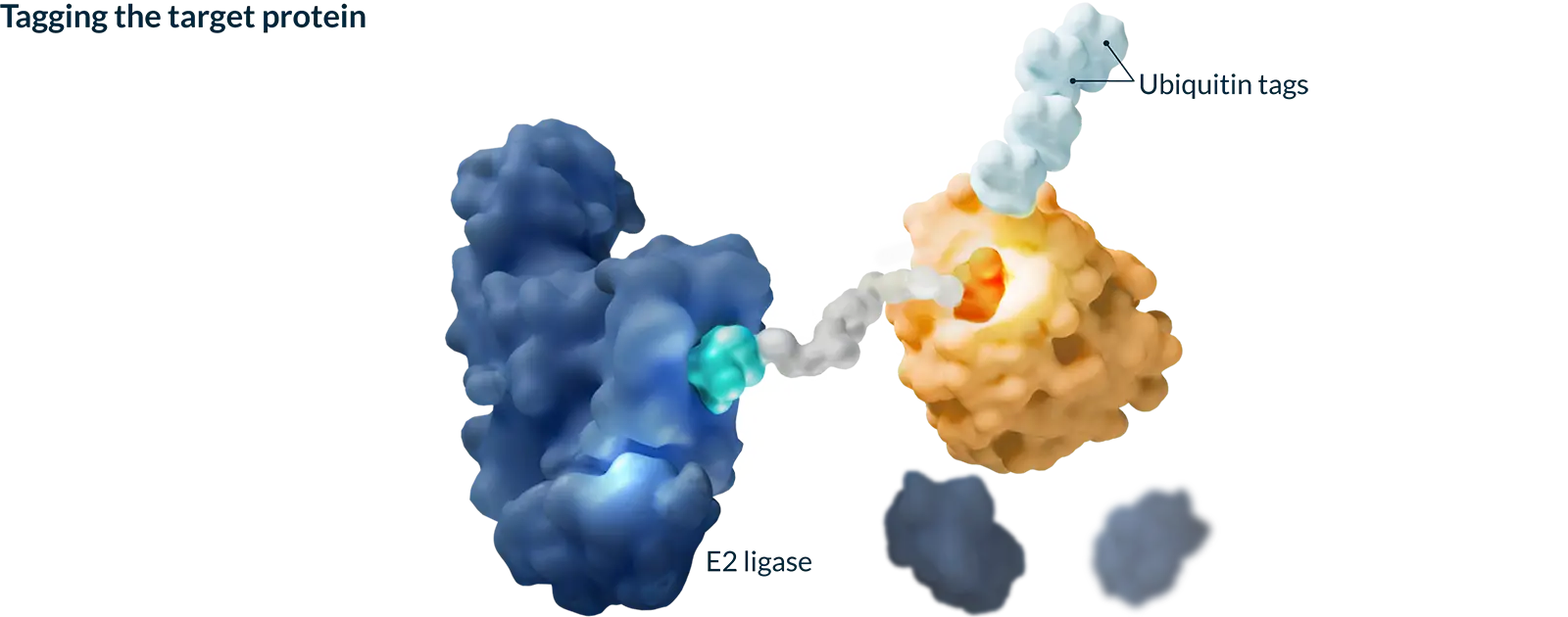
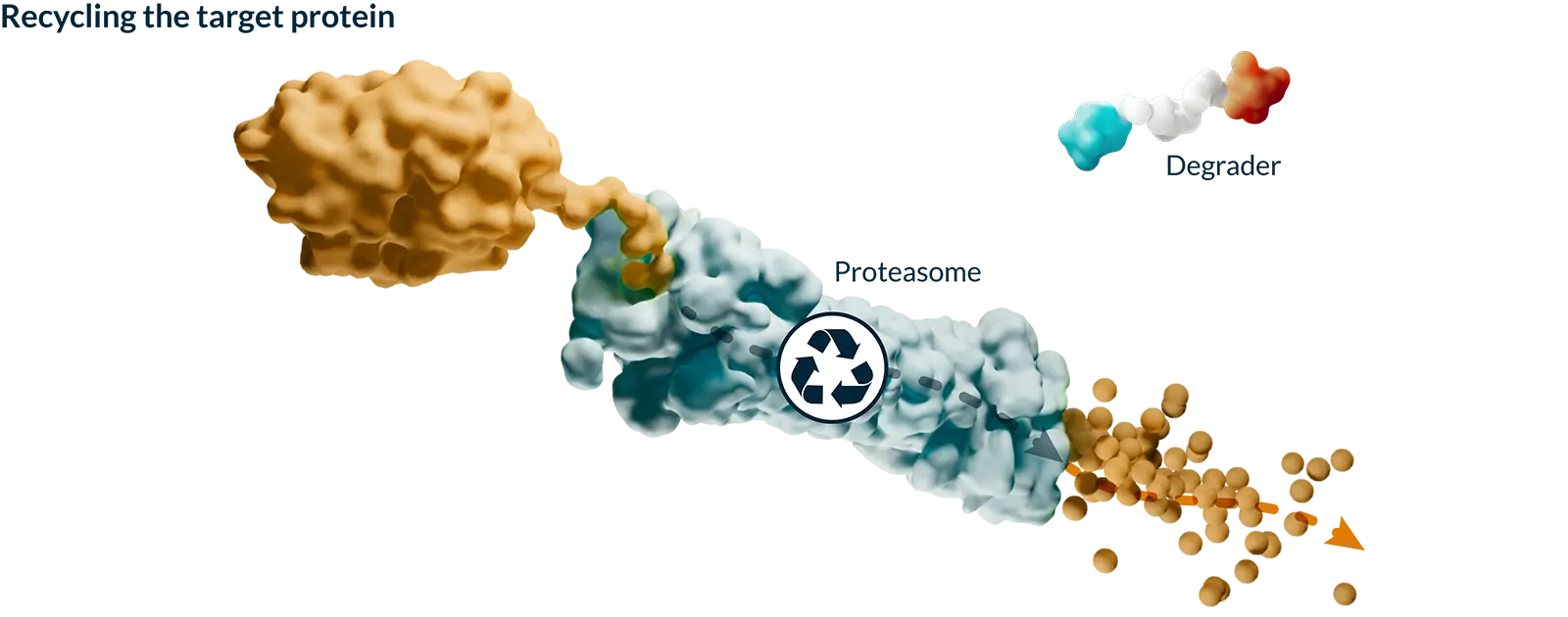
Targeted protein degradation (TPD) harnesses the natural process your cells use to keep themselves healthy by identifying and tagging misshapen or old proteins and directing them toward the cell’s built-in recycling system. Our medicines are designed to work with this system to selectively remove proteins that cause disease, with the goal of creating more effective medicines.

Clinical Trials
Clinical trials advance disease treatment by testing the safety and efficacy of the investigational medicines we are developing. Talk to your physician about whether a clinical trial or novel treatment is right for you and discover more about our ongoing trials below.
KT-621 is an investigational oral degrader in development for the treatment of immunological and inflammatory diseases
New: The KT-621 BroADen Phase 1b single-arm, open label trial is to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics, and clinical activity of orally administered KT-621 in adult patients with moderate to severe atopic dermatitis (AD).
OPEN
The Phase 1 trial is to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of orally administered KT-621 in healthy volunteers. The study includes double-blind, placebo-controlled single ascending dose (SAD) and multiple ascending dose (MAD) cohorts.
OPEN
Kymera’s investigational drugs are currently at a stage in development where we are focused on enrolling patients in our clinical trials and continuing to learn more about our investigational drugs’ safety and efficacy. At this time, Kymera is not able to make expanded access available to its investigational drugs. For more information, please read our full expanded access policy.