Targeted Protein Degradation Comes of Age
This blog was written by Nello Mainolfi, CSO and co-founder of Kymera Therapeutics, as part of the From The Trenches feature of LifeSciVC.
Translation of well validated biology into therapeutics remains one of the biggest challenges of modern drug development, in many cases due to lack of appropriate technologies to drug well credentialed biological targets. Examples in this category include driver oncogenes such as MYC, b-catenin and STAT3; catalytically active scaffolding kinases such as IRAK4 and RIPK’s or proteins whose accumulation is associated with well-established pathology such as alpha-synuclein in Parkinsons’s Disease or Tau in dementia and Alzheimer’s Disease.
Over the past decade, several new drug discovery technologies have been proven successful in overcoming some of the challenges of traditional small molecules therapeutics. One of the most successful modalities, therapeutic antibodies have allowed us to target cell surface and circulating proteins, very specifically, with high degree of affinity and most often, in a well-tolerated fashion. Not surprisingly, in any list of the most profitable drugs of the past decade (an example here) antibody based therapies (often referred to as biologics) dominate the top 10 positions. Broader applications of antibody-based therapies are limited in part by their size and properties confining them to extracellular targets (cell surface or circulating) and to parenteral administration.
Most of the potential drug targets reside within the plasma membrane of our cells, however, and many of these are not amenable to small molecule inhibitors, prompting the continued search for novel approaches to target these “undrugged” proteins.
Oligonucleotide-based therapeutics such as RNAi and antisense have provided an innovative approach to go after intracellular targets that have been technically challenging or elusive to small molecule drug discovery. Elegant and groundbreaking examples include agents such as ONPATTRO from Alnylam, the first approved RNAi therapeutics for hereditary ATTR (hATTR) amyloidosis, and SPINRAZA, an antisense oligonucleotide (ASO) that is designed to treat SMA caused by a genetic mutation that leads to SMN protein deficiency (affecting motor neurons), discovered and developed by Ionis/Biogen.
Despite the transformational nature of these new therapies, both in terms of patient impact and from a technological standpoint, broad application of oligonucleotide-based drugs is limited by drug delivery options (systemic delivery is limited to liver while local delivery is needed to achieve direct tissue targeting) as well as by dosing paradigm (parenteral administration).
The ideal solution: a small molecule-based technology, with broad systemic distribution through oral administration and the ability to drug previously un-drugged proteins with the power of knockdown/knockout approaches (like RNAi/ASO/CRISPR).
This is the value proposition of targeted protein degradation (TPD).
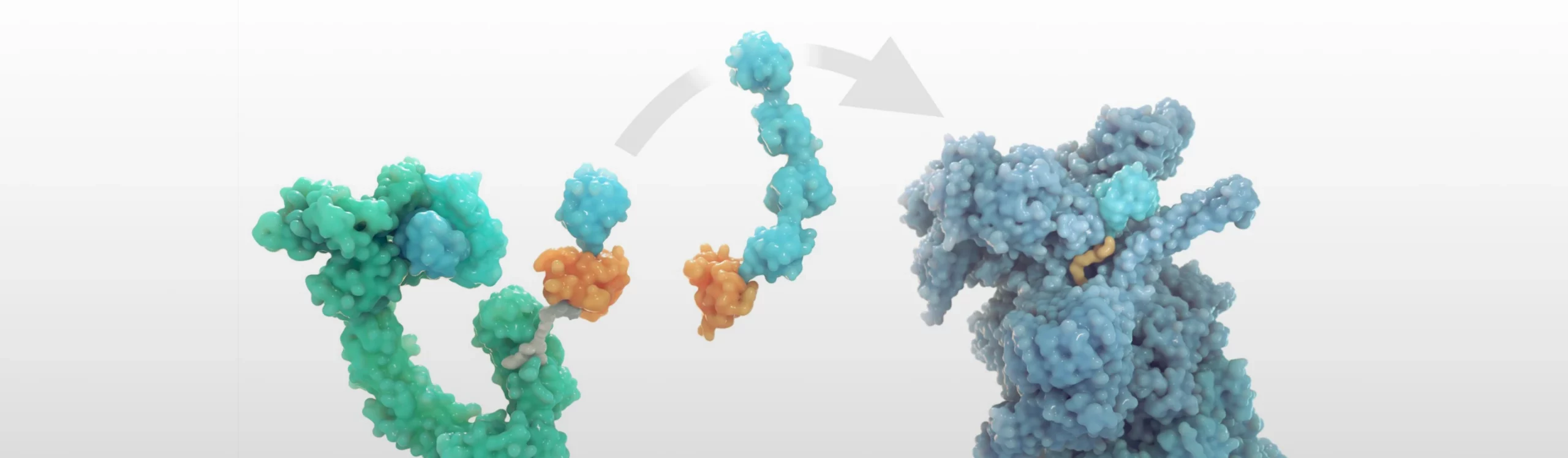
Conceptually very simple: a single small molecule simultaneously binds to a protein target of interest and an E3 ligase leading to a catalytic ternary complex that drives protein ubiquitination (through spatial proximity) and ultimately proteasomal degradation. Because of the irreversible nature of proteasomal degradation, protein re-synthesis rates dictate the pharmacodynamic response rather than the PK of the drug, the case for conventional small-molecule based approaches and most other therapeutic modalities. The history, technical aspects and recent technological advances have been well covered in a previous blog entry, in an Annual Reports of Medicinal Chemistry review as well as more recently in excellent reviews here and here.
In early 2016, I was fortunate to have the once-in-a-lifetime opportunity to co-found and build a company, Kymera Therapeutics, solely focused on translating this exciting new science to first-in-class medicines with the potential to benefit patients around the world. Very rarely does one have the opportunity to work at the forefront of completely new science – chemistry, biology and clinical experimentation – an experience that has been both exhilarating and humbling.
Kymera co-founder and Atlas Venture partner, Bruce Booth, very elegantly described the field of targeted protein degradation as well as the founding/funding thesis around Kymera in an earlier blog post which I encourage readers to reference as it provides a great context to what follows here.
Over the last 36 months, Kymera has built a protein degrader drug discovery engine – a technology platform allowing us to rationally identify targetable proteins and E3 ligases and develop drug-like degraders with the goal of advancing potent, orally-active compounds.
In just a short time, we have achieved platform proof-of-principle across a number of different protein classes. In particular, we have demonstrated preclinical proof-of-concept with our small molecule IRAK4 degraders in models of MYD88-mutated lymphoma, an aggressive form of cancer with limited treatment options. Kymera’s orally active IRAK4 degraders target both the kinase and scaffolding function, leading to complete inhibition of Myddosome signaling and superior activity vs conventional small molecule kinase inhibitors. Data around this program was presented at ASH in December of 2018 as well as AACR earlier in the year.
As a testament of our commitment to fully deliver on the promise of targeted protein degradation, we have heavily invested in capabilities to therapeutically drive degradation of traditionally “undrugged” targets – those with no catalytic function and with limited or no technical targeting options. We disclosed some of our early work against STAT3, a transcriptional regulator linked to numerous cancers and inflammatory and autoimmune diseases, earlier this year. We are rapidly advancing this project towards the clinic with plans to disclose preclinical data in 2019 at upcoming conferences and meetings.
Together with our CEO, Laurent Audoly and our management team, we have decided to focus most of our efforts in two disease areas – oncology and immunology/inflammation. These therapeutic areas represent vast opportunities for direct impact while they allow us to stay focused on our biology/disease expertise investments.
In the spirit of realizing the potentially transformational nature of protein degradation we fully believe in the ability of this platform to be target and disease agnostic.
Today, we could not be more excited to announce that we have entered a four-year strategic collaboration with Vertex Pharmaceuticals to discover and develop targeted protein degradation medicines against multiple targets of interest. Kymera will receive $70 million upfront including an equity investment and is then eligible to receive more than $1 billion in potential payments based upon the successful achievement of specified research, development, regulatory, and commercial milestones for up to six programs optioned as part of the collaboration.
More importantly, we have found in Vertex a partner that shares our core key scientific values: patient centricity, deep commitment to disease and translational biology especially with respect to target credentialing, belief in the potential of targeted protein degradation and personally a great team to interact with.
This strategic partnership, spearheaded by our CBO, Mark Nuttall, will broaden the application of targeted protein degradation to address serious diseases beyond cancer, supporting Kymera’s vision to build a platform that is disease agnostic and delivers the broadest possible impact.
Kymera is continuing its work to solve some of the typical challenges of advancing a completely new drug modality. We are working on key fundamental aspects of this technology, few of which are:
- What makes a protein specifically ligandable?
- What makes a target degradable in a therapeutically relevant setting?
- How do we appropriately degrade difficult-to-ligand targets?
- How can we degrade a target of interest only in cell types that are therapeutically relevant?
We are fortunate to have investors and partners that believe in the power of answering these key questions, because we all together believe that only by doing so, we will be able to effectively and efficiently (also in terms of capital) develop new degrader drugs.
As we are approaching clinical entry of our pipeline (2020), we have the confidence of a clinically and commercially validated modality thanks to the great work from our colleagues at Celgene (revlimid and others IMiD’s). As early champions of a transformational new technology, we are excited to see other protein degradation companies advancing degraders in the clinic, and are reinvigorated by the interest both scientific and financial to continue to invest in and advance this new class of therapeutics.