
From Target Selection to POC in Patients: Completing the Initial Human Translation of STAT6 Degradation
For as long as I can remember, I have been fascinated by solving complex problems. I can’t say that I have always solved them, but it’s been a constant refrain in my life—that feeling of comfort in being uncomfortable. I would even go as far as saying that I have always felt extremely uncomfortable when I am comfortable.
So how does this apply to Kymera? And to our programs and strategy?
We decided early on, almost 10 years ago when we founded Kymera, that the mission of the company would be to marry, as best as we can, the power of Targeted Protein Degradation (TPD) with the right target, the right disease and the right patient. Specifically, we decided to focus our research on targets that were either undrugged, or poorly drugged, and where TPD would be either the best or only modality/solution.
There has been nothing more exhilarating than working on first-in-class chemistry, biology, translational, and clinical problems. Every day is consequential – to the understanding, to the problem solving and to defining new rules. We do this with the sometimes-uncomfortable sense that while we may not know or have every piece of information, we have unwavering confidence that we have the team, the capabilities and the winning mentality to figure it all out.
In the past nine and a half years, we, the amazing team at Kymera, have built capabilities, established new drug development principles, refined human translation and built a world class team underpinned by the most crucial operating principle at Kymera: our culture of excellence. This will be a topic for another day, but in a few words, we have tried to crystallize and codify how we operate – the behaviors, principles and rules of engagement to continue to keep Kymera on the trajectory of a best-in-class biopharma company.
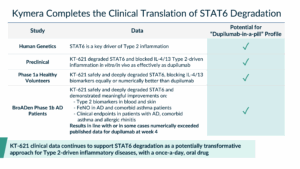
As described in previous blogs here and here, STAT6 has always represented the perfect target for Kymera:
- Historically undrugged transcription factor;
- Compelling human genetics in Type 2 allergic diseases;
- Clinical pathway validation by upstream biologics (e.g., dupilumab); and
- Compelling market opportunity to dramatically expand access to an addressable population of >100M patients worldwide. To understand the scope of the opportunity it’s important to remember that the market leader, dupilumab, is already a $20 billion drug even with just single digit market penetration for all moderate to severe Type 2 diseases
Since we disclosed our effort in this space in January of 2024, we have demonstrated that KT-621, our first-in-class oral STAT6 degrader and the first STAT6-targeted agent in clinical development with data now in both healthy volunteers and patients with atopic dermatitis, can block the IL-4/IL-13 pathway completely and at least as robustly as an upstream biologic like dupilumab. We have shown this in preclinical studies, in healthy volunteers, and now in patients with AD.
The most simple and effective way to articulate the target product profile and potential impact of KT-621 is “dupilumab-in-a-pill”. Data from our program have shown just that and more.
When we tested KT-621, a highly specific and picomolar degrader (DC90<100 pM) of STAT6, in cellular systems, we showed that it was able to block IL-4/IL-13 signaling completely and generally more potently than dupilumab, demonstrating that our STAT6 degrader blocks this pathway effectively and completely.
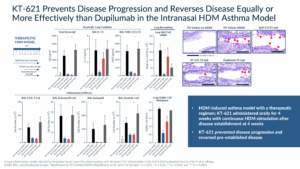
When we tested KT-621 in in vivo models of Type 2 inflammation, such as the house dust mite (HDM) asthma model, we demonstrated, in a complex and highly translatable model that STAT6 degradation, led to a profound blockade of all Type 2 relevant cytokines which resulted in robust impact on disease measurements, such as goblet cell metaplasia. In both biomarkers and efficacy endpoints, the dose that led to ≥90% degradation resulted in reductions that were in line or superior to the dupilumab arm in the same study.
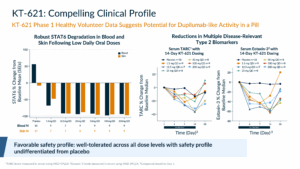
In healthy volunteers, we showed that KT-621 could degrade STAT6 in blood and skin at once-a-day doses as low as 1.5 mg. At doses above 1.5 mg, the degradation was at least 90%. We also demonstrated that STAT6 degradation led to impact of Type 2 biomarkers such as TARC and Eotaxin-3 in blood that were at least in line with, if not numerically superior to, dupilumab data in healthy volunteers. Importantly, as we had seen during all safety studies in preclinical species, we had a pristine safety profile that was undifferentiated from placebo.
Obviously the most important translation of a therapeutic hypothesis is the patient one.
We reported data on our BroADen Phase 1b study on December 8th, 2025. KT-621 was dosed once-a-day for 28 days in 22 patients with moderate to severe atopic dermatitis (AD). Ten patients received the 100 mg dose, and twelve patients received the 200 mg dose. We chose two doses that deeply degraded STAT6 in blood and skin in healthy volunteers to evaluate the fidelity of translation into AD patients. Also, given that we expected the degradation to be similar if not equal, we hoped that two cohorts could be used as each other’s internal control.
What we observed in this study went above and beyond our best-case expectations.
The two doses, as predicted and desired, translated in patients with the high fidelity we have seen in other programs. As in healthy volunteers, both 100 mg and 200 mg of KT-621 resulted in the same degradation in blood (98%) and skin (94%) with multiple subjects’ STAT6 levels below the lower limits of quantification.
With two doses that produced the same maximal pharmacological effect, we anticipated observing robust effects on biomarkers and clinical endpoints. Importantly, given the absence of a placebo arm, we expected and hoped the two doses would perform similarly enough that it would be difficult to attribute any observed effects to chance or a placebo effect.
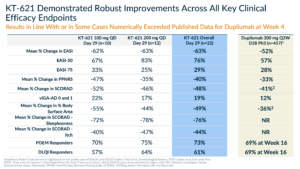
As mentioned earlier, what we observed went beyond our highest expectations. Across all biomarkers, clinical endpoints, patient-reported outcomes, quality of life measures, and comorbidities, every measure demonstrated a very strong effect consistent with a drug that profoundly impacts Type 2 inflammation. Importantly, all the results were comparable to or in some cases numerically exceeded the data reported for dupilumab at week 4.
We measured well characterized biomarkers, like TARC, Eotaxin-3, IgE, as well as others that we measured for the first time in AD studies, like IL-31 and FeNO (Fractional exhaled nitric oxide).
As the table below shows, we demonstrated effects that were at least comparable to the market leader in Type 2 diseases. FeNO deserves a special mention. It is produced by airway epithelial cells in response to Type 2 inflammation. In asthma, FeNO is a point of care biomarker used to detect Type 2 airway inflammation that has utility in diagnosis and in monitoring response to therapy with biologics, like dupilumab, targeting the IL-4/IL-13 pathway. Higher levels are associated with lower lung function and increased risk for future asthma exacerbations. We demonstrated for the first time that FeNO is elevated in patients with AD, and KT-621 can reduce it meaningfully.

The impact on clinical endpoints was very rapid, as early as Day 8, with a slope that had no apparent plateau at Day 29, implying a potential for deepening of effect with continued dosing. The robust impact that KT-621 had on AD lesions, itch, sleeplessness and other quality of life measures speaks volumes to the consistency of an effect that is in line or numerically superior to other drugs in this pathway.
A particularly compelling aspect of KT-621’s profile was the remarkable consistency of its therapeutic effects on comorbid conditions frequently presented in AD patients. Among patients with concurrent asthma, KT-621 achieved a reduction in FeNO levels exceeding 60% within the first four weeks—a result not previously observed with any other drug in this pathway. Furthermore, all patients in this group demonstrated a 100% response rate on the Asthma Control Questionnaire-5 (ACQ-5), a widely used measure of asthma disease burden.
In addition, AD patients with concurrent allergic rhinitis also experienced a very robust and clinically meaningful improvement, further highlighting the broad and reliable impact of KT-621 across multiple Type 2 immuno-inflammatory diseases.
In summary, in this Phase 1b study, KT-621 demonstrated deep degradation in blood and skin with strong translation from the Phase 1a healthy volunteer study. KT-621 had a profound effect on disease-relevant Type 2 inflammation biomarkers in blood, skin and lungs reflecting systemic IL-4/IL-13 pathway inhibition and robust clinical activity in AD with a dupilumab-like effect on all measured endpoints as well as early evidence of activity in comorbid asthma and allergic rhinitis based on improvements in biomarkers and/or patient-reported outcomes; and excellent tolerability with a safety profile similar to what was observed in the Phase 1a healthy volunteer trial.
It is worth taking a moment to reflect on what has been an exceptionally encouraging safety profile for this historically undrugged target as we have progressed KT-621 through preclinical and early clinical testing. Throughout all of our preclinical testing, up to and including 4-month tox studies in multiple species, we have not observed any safety findings at any dose. And in over 200 combined healthy volunteers and patients, there have been no reported safety issues of any significance.
I have rarely seen a study of 22 patients produce such a rich, consistent, and coherent dataset. The near-identical degradation profiles across both doses produced the same biological and clinical effects, as hoped, with consistency observed across AD and other comorbidities. Together, these results give us strong confidence as we continue investing in our Phase 2b AD and asthma programs and, following the recent FDA Fast Track designation for KT-621 in AD, accelerating our late-stage development plans across multiple indications.
I wanted to conclude by sharing a message I received last week from someone in the industry. This was the best email of the week and says it all—why Kymera and the KT-621 program exist: “…I wanted to send you a quick note to let you know that we talk about Kymera often in my house. My 5-year-old has been on dupi for 2 years, and every month, the injection is a struggle. I’ve been using the Kymera story to tell him about how new drugs are developed and why there’s hope for an oral medication to help his severe AD. I keep him updated with each new milestone for 621! So keep fighting the good fight! I’m rooting for you!”
Stories like this reinforce our commitment to fight for patients, to turn scientific milestones into real patient impact, and to never lose sight of the people who are counting on what we do.
Nello Mainolfi, PhD
Founder, President and CEO
Kymera Therapeutics